Abstract
Myeloproliferative neoplasms (MPNs) are clonal stem cell neoplasms that include polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). MPN patients are immunocompromised due to immune dysregulation from clonal hematopoiesis, heightened inflammation, and immunosuppressive treatments. Infection is a leading cause of morbidity and mortality in MPNs, and as a result annual influenza vaccination is a critical component of care. However, efficacy of the influenza vaccine in MPN patients is unclear. This study sought to identify differences between MPN patients and healthy donors in their immune response to influenza vaccination and subsequent in vitro stimulation.
The Massachusetts General Hospital (MGH) maintains a tissue bank of peripheral blood and bone marrow samples collected in consented patients with hematologic malignancy, including MPNs. The tissue bank was interrogated for MPN patients with peripheral blood samples banked 1-6 months after yearly trivalent/quadrivalent influenza vaccination. A total of 13 MPN patients were included, with a median age of 70 (32-86). Five patients had MF, 3 patients had PV, 4 patients had ET, and 1 patient had pre-fibrotic MF. Five patients were on treatment with ruxolitinib, 4 patients were on hydroxyurea (HU), and 4 patients were without treatment. Peripheral blood mononuclear cells (PBMCs) from 11 healthy donors 3-6 months post-vaccination, with a median age of 50 (28-56), were sourced commercially as controls.
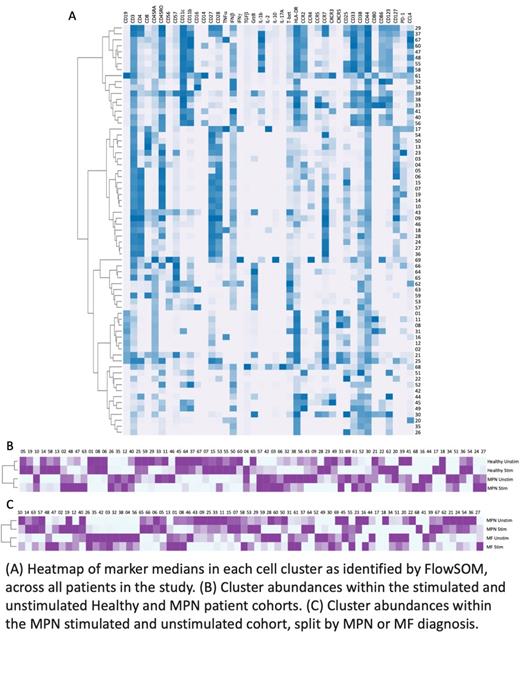
Cryopreserved samples were thawed and PBMC samples divided equally prior to incubation for 18 hours with peptides derived from Influenza A or sham. Subsequently samples were labeled with a 41-antibody panel and analyzed by mass cytometry. Manual gating together with FlowSOM clustering followed by EdgeR using T-test and FDR to correct for multiple comparisons were used to identify differentially abundant immune cell clusters between cohorts and treatment conditions.
Without stimulation, MPN patients were decreased in B cell and memory CD4+ T cell populations as compared to healthy donors (p<0.05), in agreement with a previous report. MPN patients also exhibited an increase in a subset of mature B cells (p<0.05) and lineage negative CD123+ cells (p<0.05) as compared to controls. Stimulation included in this study aimed to expand on these distinctions and identified that baseline differences were retained with additional increases in CD4+ and CD8+ T cell responses in healthy donors as compared to MPN patients. Conversely, stimulation increased the frequency of inflammatory monocytic cells in MPN patients compared to healthy controls (p<0.05).
In healthy donors stimulation induced several alterations in, most notably an increase in CD4+ effector memory cells (p<0.03), as compared to matched unstimulated controls. In contrast, comparison of pre- and post-stimulation within the MPN cohort revealed no significant differences in immune cell populations apart from a decrease in naïve CD4+ T cells (p<0.05). Patients receiving HU were enriched for naïve CD4+ and effector CD8+ T cells as compared to patients receiving ruxolitinib (p<0.05); however, these differences were diminished following stimulation. Within MPN patients, pre-stimulation samples in MF patients were characterized by increased abundance of activated monocyte/macrophage clusters and a lineage-negative CD123+ population; there were no significant differences in post-stimulation samples between MF and ET/PV/pre-fibrotic MF patients.
We evaluated immunologic responses to influenza vaccination, with and without peptide stimulation, in MPN patients and compared to healthy controls. Overall, MPN patients demonstrated less robust B- and T-cell responses and a muted response to stimulation as compared to healthy controls, which may indicate reduced cellular responses following influenza vaccination. Ongoing analysis will interrogate specific immune populations and subsets in detail to refine current observations and will evaluate associations between immunologic cell clusters with serologic responses and additional patient characteristics. The results of our study highlight important alterations in the immune response to influenza vaccination and muted response to stimulation in MPN patients and will inform a future prospective study of vaccine responsiveness and immune dysfunction in patients with MPNs.
Reeves: PR Fludigm: Honoraria; Immunoscape: Consultancy. Hobbs: Merck: Research Funding; Incyte Corporation: Research Funding; AbbVie.: Consultancy; Novartis: Consultancy; Celgene/Bristol Myers Squibb: Consultancy; Constellation Pharmaceuticals: Consultancy, Research Funding; Bayer: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal